Cytoplasmic Transfer
Cytoplasmic transfer is a method of transferring a cell’s cytoplasm from one cell to another. Although this method has many advantages, it can also be a disadvantage. Some strains of mice halt embryonic cleavage early, so cytoplasm from a non-blocking strain can help overcome this problem. However, cytoplasmic transfer can cause embryonic wastage in the DDK strain. This is because of cytoplasmic incompatibility.
PB1T
The PB1T procedure is a recombinant DNA technology involving the transfer of the nDNA from one fertilized oocyte to another. Generally, the procedure is performed using embryos with a small amount of cytoplasm and mtDNA. General information about the PB1T procedure can be found below. Although a significant percentage of patients will experience a positive outcome, there are risks associated with this treatment.
This procedure is similar to the MST and PNT procedures, and involves the transfer of the first polar body to an oocyte. This method is less rigorous than either MST or PNT. However, it has not been shown to prevent the transmission of mtDNA disease, and more research is needed to confirm its safety and effectiveness. In the meantime, there is no evidence that PB1T is an effective method of preventing the spread of mtDNA disease.
Ooplasmic transfer
The Methodology and Procedure of Cytoplasmic Transfer is a new method of transferring oocytes from mother to child. It was first developed in 1982 by Audrey Muggleton-Harris at the MRC Laboratory Animals Center in Surrey, United Kingdom. The first successful ooplasmic transfer was recorded in mice in June of that year. In 1998, the FDA notified US fertility clinics and held a public meeting on the method. A statement by the agency noted that two dozen births had been attributed to this technique since 1998.
In addition to improving fertilization rates and the development of early embryos, cytoplasmic transfer has also been shown to delay the need for egg donation. As of this writing, nearly thirty healthy children have been born worldwide using this method. Despite the risks and complications of this procedure, it is an extremely effective treatment for poor-quality oocytes and is recommended by many fertility specialists. Although a few of these children were born with disabilities or other complications, the vast majority of CT patients were healthy and born in the first try.
Pronuclear transfer
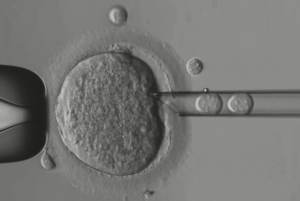 The pronuclear transfer procedure of cytoproteinic transfer uses an egg from one woman to produce a new embryo. The cytoplasm is a part of the egg and is not visible with the naked eye. Scientists have described this technique in two different ways. One method uses a fertilised egg from another woman, and the other uses a polar body. During the menstrual cycle, the egg is released into the uterus and is a secondary egg. These eggs contain mostly nuclear DNA and little cytoplasm, which contains mitochondria.
The pronuclear transfer procedure of cytoproteinic transfer uses an egg from one woman to produce a new embryo. The cytoplasm is a part of the egg and is not visible with the naked eye. Scientists have described this technique in two different ways. One method uses a fertilised egg from another woman, and the other uses a polar body. During the menstrual cycle, the egg is released into the uterus and is a secondary egg. These eggs contain mostly nuclear DNA and little cytoplasm, which contains mitochondria.
A similar procedure called spindle transfer achieves similar results, but is more expensive. It involves the excision of the metaphase II spindle from the donor oocyte, which provides the cytoplasmic constituent to the embryo. In addition, the donor nuclear DNA is contained in the karyoplast. The intended mother’s oocyte is then removed, and the chromosome-spindle complex is transferred to a healthy donor oocyte.
Polar body 2 transfer
A polar body 2 transfer is a type of cytoplasmic transfer. It involves the transfer of a second polar body from an oocyte that has been fertilized with a partner’s sperm. The polar body 2 contains the genetic material from the partner. It is possible to reconstruct an embryo from two donor oocytes and a single donor oocyte. After fertilization, the second polar body extrudes. The donor oocytes and the zygote form two embryos, and one has a single male pronucleus.
The polar body is not a reliable landmark, and the cellular content of an egg varies widely depending on its species. Hence, different studies have reported varying levels of carry-over. For instance, ST allows a very low amount of mtDNA transfer, whereas PBT enables a minimum amount. A polar body is not an infallible landmark, and a polar body may contain endogenous retroviral proteins.
Follicular aspiration
The procedure of cytoplasmic transfer, also known as ICSI, involves transferring a small amount of cytoplasm from a donor oocyte into an incompetent oocyte. This technique has been studied for both its benefits and risks. Although it has been associated with some abnormalities in the resultant children, these are not likely to be caused by the procedure itself. Clinical applications of this procedure were developed before extensive animal studies were completed. Tang et al. found that cytoplasm transfer was effective for the transfer of oocytes to infertile ovules.
PNT and ST may help to prevent embryo aneuploidies. These problems are often a result of the poor cytoplasmic quality of oocytes from elderly mothers. While both procedures can increase the fertility of the reconstructed oocyte, the results of both procedures should be interpreted carefully. In addition, they can lead to euploid embryos. Thus, it is important to have a healthy embryo for a child’s development.
Polar body 3 transfer
A polar body is a single cell that contains complementary chromosomes to an oocyte. It is a viable option for assisted reproductive technology (ART) and has several benefits. In 1990, polar body biopsy was first performed for preimplantation genetic diagnosis. This technique involves the removal of PB1 from an unfertilized oocyte and transferring it to a donor oocyte. After fertilization, PB2 is removed from the donor oocyte and substituted for the female pronucleus of a donor zygote.
A polar body biopsy is the first step in the procedure. The oocyte is mechanically dissected to harvest polar body cells. After harvesting the cells, they are fixed in a fixative (methanol-glacial acetic acid, 3:1), which is stored in the freezer until needed. Before transferring the polar body, the patient must have a glass microscope slide with a circle engraved on it with written information. The slide must be pre-cleaned with fixative and purified water.
mtDNA
The procedure of cytoplasmic transfer (CT) is a technique in assisted reproduction that can prevent transmission of diseases affecting mitochondrial DNA. The procedure was developed to treat infertility in women over 35 years old, but the benefits of CT were not fully understood. There were few human studies to prove the safety of this procedure, and few experiments were performed on animal models. Likewise, the health implications of heteroplasmy (transfer of donor mtDNA) were not understood. Animal studies that investigated mitochondrial replacement produced conflicting results. Some animals experienced accelerated aging and degraded cognitive function. Others were healthy.
There have been reports of successful ooplasmic transfer, but these cases are rare. In theory, the donated ooplasm provides the embryo with mitochondria and other factors that support development, but some studies have reported an increased risk of birth defects and chromosomal abnormalities. These studies include the work of Jacobs and Brown. Nonetheless, there are concerns about the safety of PNT in infertile women.
mtDNA synthesis
MMT, or maternal mtDNA synthesis procedure, is a regenerative therapy in which the embryonic stem cell (ESC) or karyoplast (the cellular component of a cell) is implanted into a recipient oocyte. The procedure is successful, and it leads to comparable development of the blastocyst compared to control ICSI embryos. The resulting embryos yield two novel ESC lines and four healthy infants. The procedure has two major side effects: the transfer of the cytoplasmic material can cause respiratory distress in the fetus.
Until now, there have been no widely-approved treatments for mitochondrial disease. Current therapies only manage to control its symptoms and slow its progression. The only way to completely prevent mitochondrial disease is to understand how mtDNA mutations affect the cell’s metabolism. Molecular genetics has shown that mitochondrial diseases are associated with defects in the mtDNA synthesis procedure. As such, the aim of the mitochondrial screening therapy is to protect offspring from passing on the disease.